Gold (III) bromide Formula
Gold (III) bromide, also known as gold tribromide and more frequently as auric bromide, is an inorganic salt of gold mainly used as catalyst in organic and inorganic synthesis.
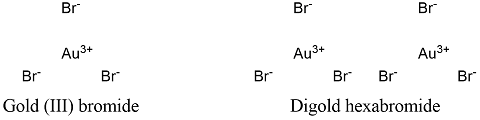
Formula and structure: Gold bromide chemical formula is AuBr3 and its molecular weight is 436.69 g mol-1. The molecule is formed by a gold cation Au3+ and 3 bromide anion Br -, that are bounded by ionic bonds. The gold (III) bromide structure is mostly found as a dimer of digold hexabromide. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Gold bromide is not found in nature. It is only prepared from gold and bromine, a synthetic method that was invented in the 19th centry.
Preparation: Gold bromide is prepared from its basic component: metallic gold and liquid bromine. There are two very used methods to obtain gold bromide; the first one is the simple reaction with gold and bromine at 150 ºC (reaction I) and the second methods is the reaction with gold (III) chloride and hydrobromic acid (reaction II):
2 Au + 3 Br2 → Au2Br6
Au2Cl6 + 6 HBr → 6 HCl + Au2Br6
Physical properties: Gold bromide is a blue to grey or black crystalline solid or powder. Its density is 5.92 g mL-1 and the melting point is 97.5 ºC and in higher temperature it decomposes. It can also decompose when reacts with glycerol. It is slightly soluble with water and ethanol.
Chemical properties: Gold (III) bromide is a Lewis acid, so that it is a chemical specie that accepts electrons and it is probably the reason why the gold (III) bromide is only found as a dimer of Au2Br6. The coordination of two AuBr3 molecules forms a most stable structure than a monomer of the salt. However, the AuBr3 molecule can also be found in the hydrate form. Moreover, the gold (III) bromide can also reacts with Lewis bases such as hydrobromic acid:
HBr + AuBr3 → H+AuBr4-
Uses: Gold (III) bromide is a popular catalyst use in chemical industry and inorganic and organic synthesis where it is apply due its characteristic of Lewis acid. It is used in medicine, natural products research and in the criminalist field to test fluid spermatic and alkaloids.
Health effects / safety hazards: Gold (III) bromide is toxic and can cause damage by ingestion. It is also an irritant of eyes and mucosa. It is not flammable and it is relative inert to other chemical compounds.
|
Related Links: |
